 Finn Chambers on Scanpor are intended for use by or under the supervision of a physician.
Finn Chambers on Scanpor are intended for use by or under the supervision of a physician.
Finn Chamber® is a patch test device which provides good occlusion because of the chamber design. Made of aluminium, the 8 mm inner diameter provides a 50 mm² area and about 20 µl volume. Larger chambers (12 mm inner diameter) and chambers with polypropylene coating (8, 12, 18 mm) are available for special purposes. Finn Chambers are mounted on Scanpor® tape from Actavis Norway AS, Norgesplaster, with protective paper backing which is easily peeled away.
Finn Chambers on Scanpor are available in strips of 10 (2x5), 5 (1x5) and 1 chambers. The strips of 10 chambers are practical when testing with a large number of substances, e.g. with routine tests. Smaller strips are suitable for small test series and individual tests. The strips should not be cut between the chambers, as the remaining adhesive area may be too small.
For locating the test sites a special device, Reading Plate, is recommended. Finn Chamber Tray aids the handling of test tapes at the application of test substances.
Allergic reactions to aluminium and Scanpor tape are rare. However, occasional cases of contact sensitivity to aluminium e.g. due to vaccination or hyposensitization of hay fever patients with aluminium precipitated antigens have been reported.
The skin may react to the removal of the tests with a slight mechanical irritation, indicated by erythema on the area covered by the tape.
Test substances are usually applied in petrolatum. The concentrations of allergens in most standard series are suitable for Finn Chambers. When using uncommon test substances the administering physician should choose carefully the substances and concentrations. It is advisable to use low concentrations with irritating test substances due to tight occlusion provided by the chamber.
Due to the incompatibility of aqueous mercuric solutions with aluminium, polypropylene coated Finn Chambers should be used when testing mercuric compounds. Aluminium may enhance the polymerization of acrylate monomers and false negative reactions have been noticed with acetone solutions of ethyl cyanoacrylate glue.
Instructions for use
 Finn Chambers on Scanpor are intended for use by or under the supervision of a physician.
Finn Chambers on Scanpor are intended for use by or under the supervision of a physician.
Application of the test substances
Mark identification on the top of each tape to show the order of the test substances throughout the testing procedure. Remove the protective paper and place the tape on the desk or the Finn Chamber Tray with the chambers up. Keep a narrow strip of the protective paper on the tape until the tape has been attached onto the skin.

1. If you put the tapes with the marked end to the left, you can fill the chambers in the normal reading order from left to right.
2. In order to provide non-sticky handling of the tape keep the narrow strip of the protective paper on the tape until the tape has been attached onto the skin. The removed protective paper can also be reattached on the edge of the tape.
 Semisolids (e.g. petrolatum as the vehicle) are applied directly into the chamber, filling
more than half the chamber volume (a bar of about 5-6 mm if the diameter is 2 mm). Do
not use filter paper discs with semisolids.
Semisolids (e.g. petrolatum as the vehicle) are applied directly into the chamber, filling
more than half the chamber volume (a bar of about 5-6 mm if the diameter is 2 mm). Do
not use filter paper discs with semisolids.
A toothpick is useful for removing excess of test substance from the chamber.
For liquids. place a filter paper disc in the chamber. Moisten the disc thoroughly without surplus. Excess liquid should be removed e.g. with porous paper. Place the test onto the skin within a few minutes. Do not let the filter paper disc dry, because this may result in weak or false negative reactions and the disc tends to slip out during application onto the back.
The last chambers are preferably reserved for liquid test solutions (e.g. formaldehyde in water), because this minimizes drying.
Placing the tests onto the skin
The patient shall stand or sit in a relaxed normal position, the back slightly bent forwards. The tests are applied to healthy skin on the back, which is to be free of ointments and excessive sebum. It is recommended that the patient takes a shower or bath in the morning before testing. If necessary, the skin can be cleaned e.g. by alcohol.
Apply the tape starting with the lower part and pressing the chambers from below to let the air escape. After having applied the tape this way, press each chamber containing a semisolid gently with the finger to get an even distribution of the test substance. Rub the tape gently but firmly with the palm against the skin, especially on the corners, to ensure good adherence. The patient should refrain from vigorous activities and taking showers during the first day after application of the tests.
The skin can be marked e.g. with nontoxic ink or tape. If the patient can come to the clinic for removal of the tests, the physician has the advantage of using the marked tape for locating the test sites, and no marking of the skin is necessary.
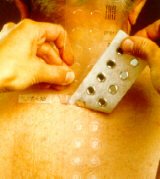 Removing the tests
Removing the tests
The tests are removed after one or two days' exposure. Whenever possible the patient should return to the clinic for removal of the tests. If the patient removes the tests himself, he should be advised to destroy the remnants properly.
Check test sites immediately after removal of the tests. A ring-shaped depression around each test verifies the occlusion and validates the test, especially in the case of negative reactions.
The serrated edge of the Reading Plate can be used to cut the tape. Press the Reading Plate against the tape at about a 45° angle with the serrated end overlapping the top 1 cm of the tape. Tear off the tape with chambers, starting from below and leaving the top of the tape with the identification marking on the skin. The serrated edge of the Reading Plate is asymmetrical; keep the wider side of the Reading Plate to the right or for sharper cutting to the left.
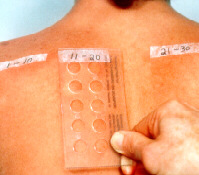 Reading the test reactions
Reading the test reactions
The tests should be read not less than 20 minutes after removal. The subsequent readings should be done 3-7 days after the application of the tests. Observing the course of the reactions helps in differentiating the allergic reactions from the irritant ones.
To locate and identify the reactions align the top left corner of the Reading Plate (narrow side to the
left) either with the ink mark on the skin or the remaining strip of the tape. The Reading Plate serves as
a template with the holes indicating the test sites.
Storage at room temperature, cold tape adheres less well.
![]()
Rannankoukku 22, FI-04300 Tuusula, Finland
tel. +358 9 275 5366, fax +358 9 275 4335
WWW: http://www.epitest.fi/ - E-mail: [email protected] - Feedback
Updated 3 February 2006.